
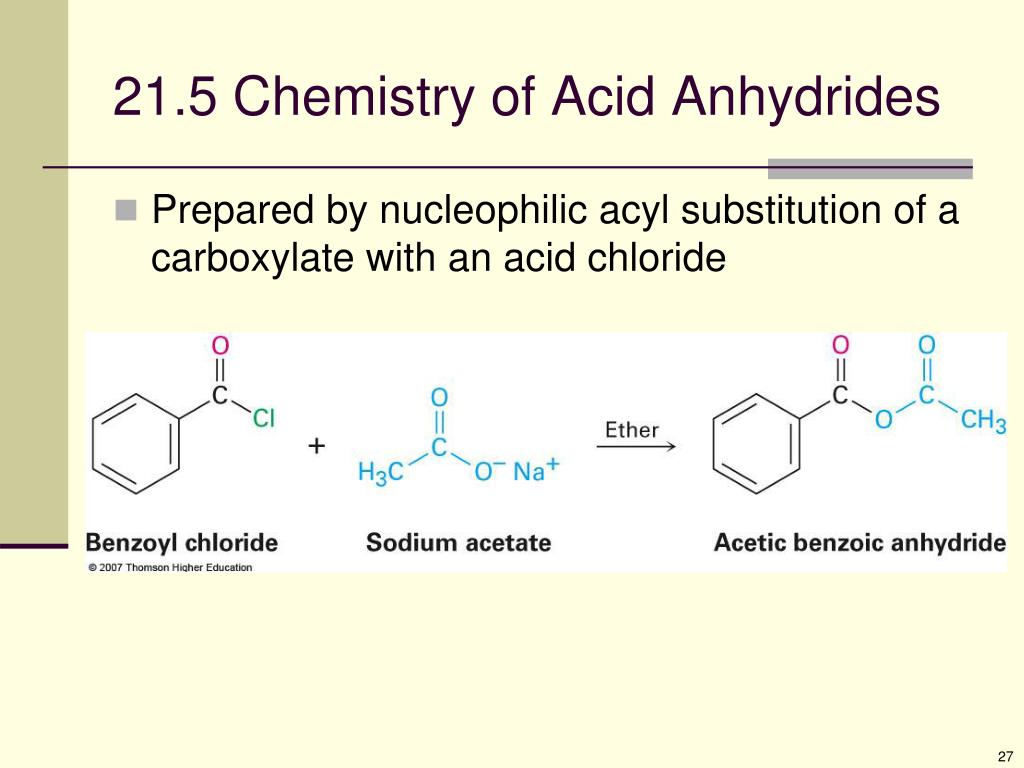
Reduction to alcohols, conversion to acyl chlorides, ester and also amides.įor sodium bicarbonate test, the bubbles of carbon dioxide were formed when sodiumīicarbonate was added to ethanoic acid. Which are salt formation that include neutralisation and reactions with electropositive metals, There are a total of five reactions for carboxylic acid The objective of this experiment is to investigate the reaction of Carboxylic acids and Write and explain all the equations involved in this experiment. Diluted and concentrated sulphuric acidĬH 3 CONH 2 + HNO 2 → CH 3 COOH + N 2 + H 2 O QUESTION.To investigate the reaction of Carboxylic acids and its derivatives with certain reagents. Among these acid derivatives are the acyl The carboxyl function is replaced by other groups. Other important classes of compoundsĬalled acid derivatives are related to acids but differ from them in that the hydroxyl portion of CarboxylicĪcids are an important class of organic compounds. (carbonyl or hydroxyl) can double bond when proton is removed from hydroxyl. Negative charge and this results in resonance stability. The carboxyl group differs from a hydroxyl group in having a delocalized Note also that the hydrocarbon part has much In water and the other not soluble in water. Part (the other five carbons with their associated hydrogen atoms). The resulting molecule then has a hydrophilic part (the carboxyl group) and a hydrophobic Is more than one, for example six, then five of the carbons are hydrocarbons and non-polar. Thus carboxylic acids form hydrogen bond with themselves and any otherĪppropriate polar molecule, and this structure will influence their physical properties and theirĬhemical properties as we have seen in other cases. The carboxyl functional group is polar, both at the carbonyl part and at the hydroxyl partīecause of the difference in electronegativity value of carbon - oxygen, and oxygen. The names of the first ten alkanes and convert these names to the corresponding carboxylicĪcid. Nomenclature drops the ( -e ) of the suffix of the Alkane name and adds ( -oic acid), review DATE OF EXPERIMENT 22 APRIL 2020 DATE OF SUBMISSION 29 APRIL 2020 INTRODUCTIONĬarboxylic acids contain one or more carboxyl functional groups (-COOH). Universiti Teknologi Mara(UiTM) Cawangan Sabah Kampus Kota Kinabalu CHM CARBONYL COMPOUND – REACTIONS OF CARBOXYLIC ACID AND ITS DERIVATIVES EXPERIMENT 3 GROUP MEMBERS


 0 kommentar(er)
0 kommentar(er)
